Akin to say food poisoning, when it comes to cramping, especially exercise-associated muscle cramping (EAMC), there are two types of people: those who have cramped and those who have yet to cramp. Exercise-associated muscle cramps are defined as painful spasms and involuntary contractions of the skeletal muscles that occur during or immediately post-exercise (12). This therefore excludes cramps occurring outside of the context of exercise or because of underlying medical conditions such as familial nocturnal cramps, endocrine-metabolic diseases such as hypo/hyperthyroidism, and central and peripheral nervous system diseases like Parkinson’s disease or multiple sclerosis (20).
To many of you, cramping is not news. It has been a topic of great debate for decades, especially in the endurance community. How do we prevent them? Stop them? Rid ourselves of them for good? Most of us have experienced mild to even debilitating cramps at some point in time, and there is nothing fun about being stuck mid-race trying to decide if crawling is a legitimate option.
In short, cramping sucks and despite our long affair with EAMC, we are not much closer to fully understanding their etiology–where they come from. If anything, our new understanding of EAMC is that they are complicated and likely stem from multiple compounding factors that make any one treatment or preventative technique unlikely to work for everyone, every time.
What has developed in the scientific community over the past five to 10 years is a clear movement away from the original dehydration/electrolyte-imbalance theory and an increased focus on the altered neuromuscular control theory. Since the early 2000s, study after study (2, 3, 4, 8, 9) emerged looking at hydration status and blood-electrolyte concentrations (primarily sodium and potassium) in endurance athletes post-competition and over and over again, there was no significant difference in the hydration status or blood-electrolyte concentration of athletes who cramped and athletes who did not cramp.
Examples of this data are shown in the graphs below comparing dehydration status and sodium intake across crampers and non-crampers in a long ultramarathon. Moreover, dehydration and electrolyte imbalances are system-wide issues and therefore should cause system-wide muscle cramping and not localized problems (17). Most EAMC primarily occur in asymmetry–one calf cramps. However if muscles are cramping bilaterally, like in both both calves, or become generalized/full-body cramping, they can be tied more closely to dehydration or a more serious medical condition. What this means is that we should not eliminate dehydration and electrolyte imbalances entirely from the EAMC playing field. Instead it’s more likely that hydration and electrolyte issues act as two of the many players that work together to cause EAMC rather than being the sole cause in most cases.
Details from many of these studies were covered thoroughly in columnist Joe Uhan’s 2013 article for iRunFar. If you haven’t read Joe’s article, consider doing so as an accompaniment to this one.
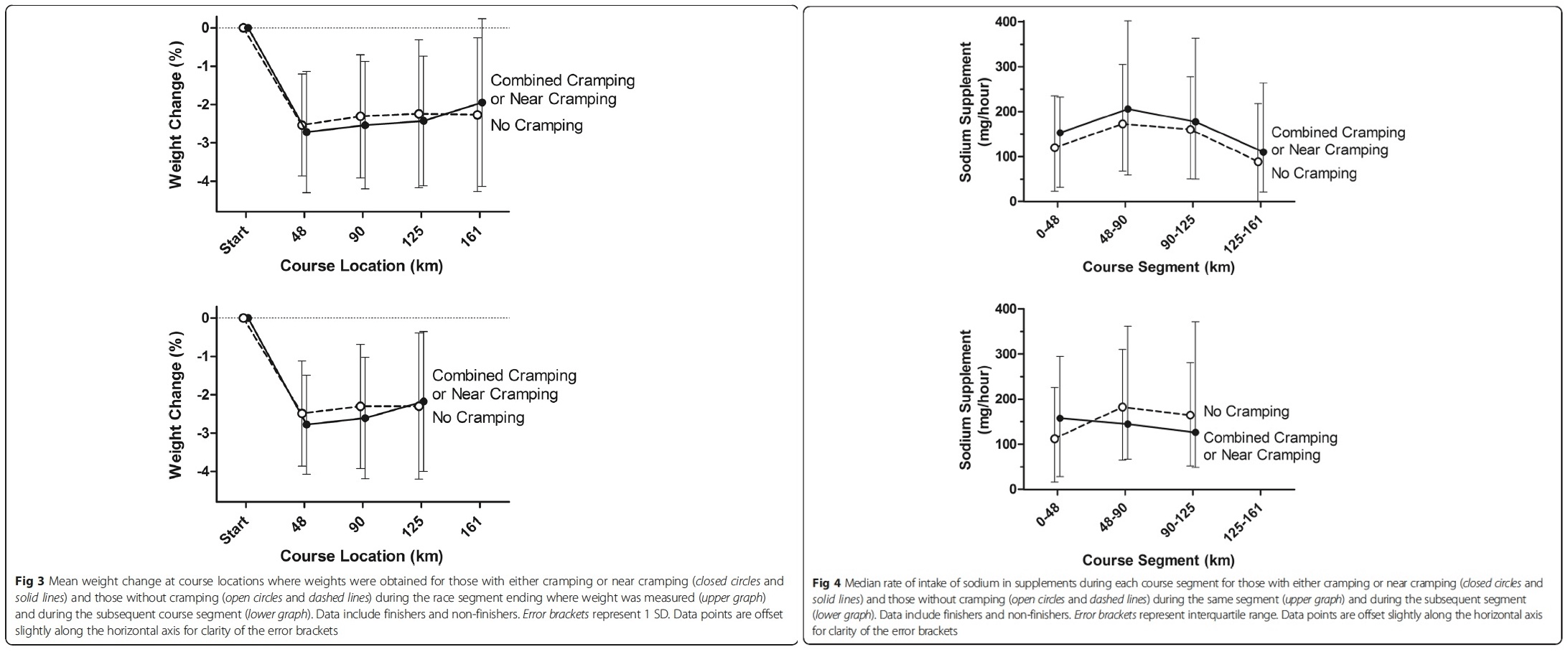
(Left) Dehydration has often been tracked by percent body weight lost during exercise. In these graphs, you can see that there is little to no difference in percent body weight lost and if the participants were cramping or not over the course (locations noted in kilometers) of a 161k race. (Right) Sodium supplements have long been used as a means of hopefully preventing cramping during ultra-endurance events. In these graphs, sodium supplements (milligrams/hour) ingested were measured multiple times during a 161k race. You can see that there is very little difference in the amount of sodium ingested per hour and if the participant cramped during the race. Images from Hoffman, M. D., & Stuempfl, K. J. (2015).
These studies suddenly opened up the door to new theories and possibilities. Chief among them was the altered neuromuscular control theory, or the idea that the root cause is coming from our nervous system’s relationship with muscle contraction. This theory suggests that EAMC are a combination of several factors coalescing in a perfect storm to over excite your alpha motor neurons resulting in, you guessed it, cramping. Alpha motor neurons are the largest neurons in your spinal cord and they are responsible for delivering the message for skeletal-muscle contractions and movement. The factors looked at most keenly are variables leading to heightened fatigue state such as: inadequate training or conditioning including improper heat and/or altitude acclimation; muscle damage; previous injury to both the cramping muscle or in compensating muscle group; and certain medications like albuterol (taken for asthma), conjugated estrogen (used during menopause), and statins (for managing cholesterol) (11). You can see how these variables build off each other in the chart below (11). These factors could also explain why EAMC seem to come on more in the heat where muscles are fatigued earlier at the same work load, and why athletes with a history of previous cramping are most likely to experience cramping in the future (5).
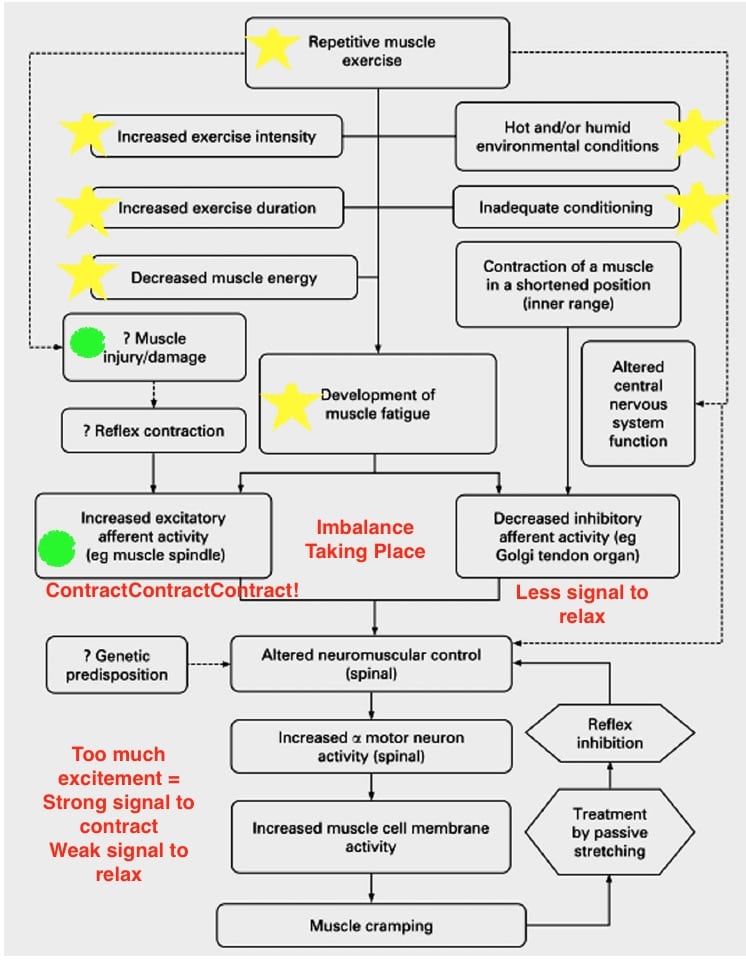
Yellow stars represent the potential variables impacting muscle fatigue. Green circles indicate additional causes of neural imbalance telling the muscle to contract more. Image from Schwellnus M.( 2009) with edits by author.
So how do we treat the onset of a cramp?
Two methodologies to treat EAMC have emerged at the forefront of the altered neuromuscular control theory. They are: targeting your Golgi tendon organs (GTO) and your transient receptor potential (TRP) channels. I realize that is a lot of information packed into one breath, but bear with me, I’ll explain more shortly, but first your GTO.
Located in your skeletal muscles, Golgi tendon organs are your very own built-in anti-cramp tool. It’s important to understand that muscle contractions are not simple, but rather the perfect coordination between our nervous system, its sensors within our muscles, and the right messages making it back forth between the two. When functioning properly, GTOs act as the inhibitor to your muscle spindles causing contractions. Your alpha motor neurons and muscle spindles–the stretch sensor in your skeletal muscle–are the active “contract-contract-contract!!!” command and action that keeps us moving down the trails while the GTOs are the inhibition to the contraction that allow the muscle to relax. As our muscles fatigue, there is an increased firing from the muscle spindles to keep “contracting-contracting-contracting!!!” while, at the same time, there is a lessened response from the muscle GTOs to relax. When both of these things happen, we get over-excited alpha motor neurons that cause the contraction to win out time and time again, resulting in a contraction that won’t stop, or as we’ve experienced, a cramp (7, 12).
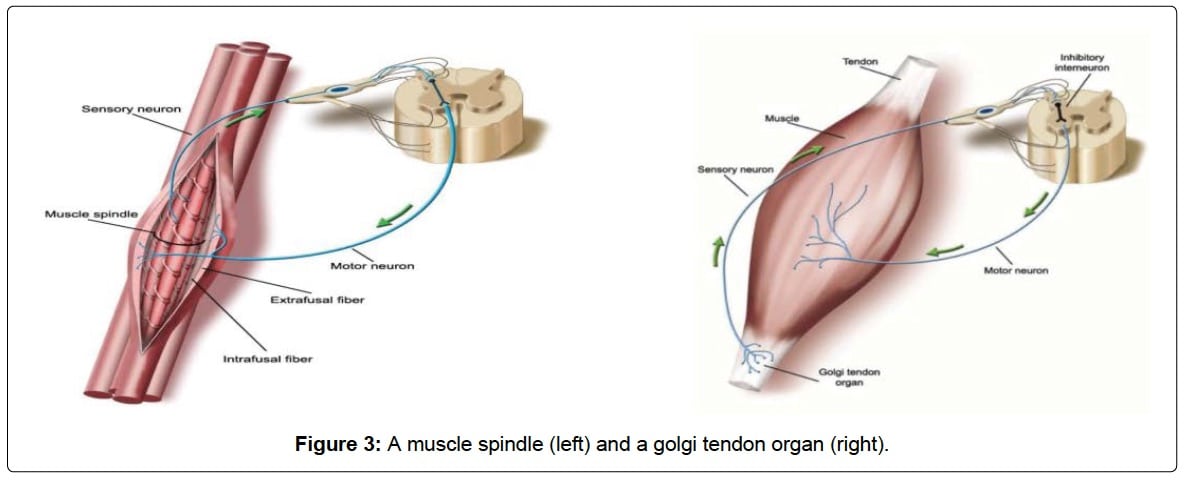
Muscle spindle (left) transmits information about muscle stretching in order to contract your muscle and protect it from stretching past its endpoint and the Golgi tendon organ (right) senses and transmits information about muscle tension in order to signal your muscle to relax (inhibits contraction). Image from Qiu J, Kang J (2017).
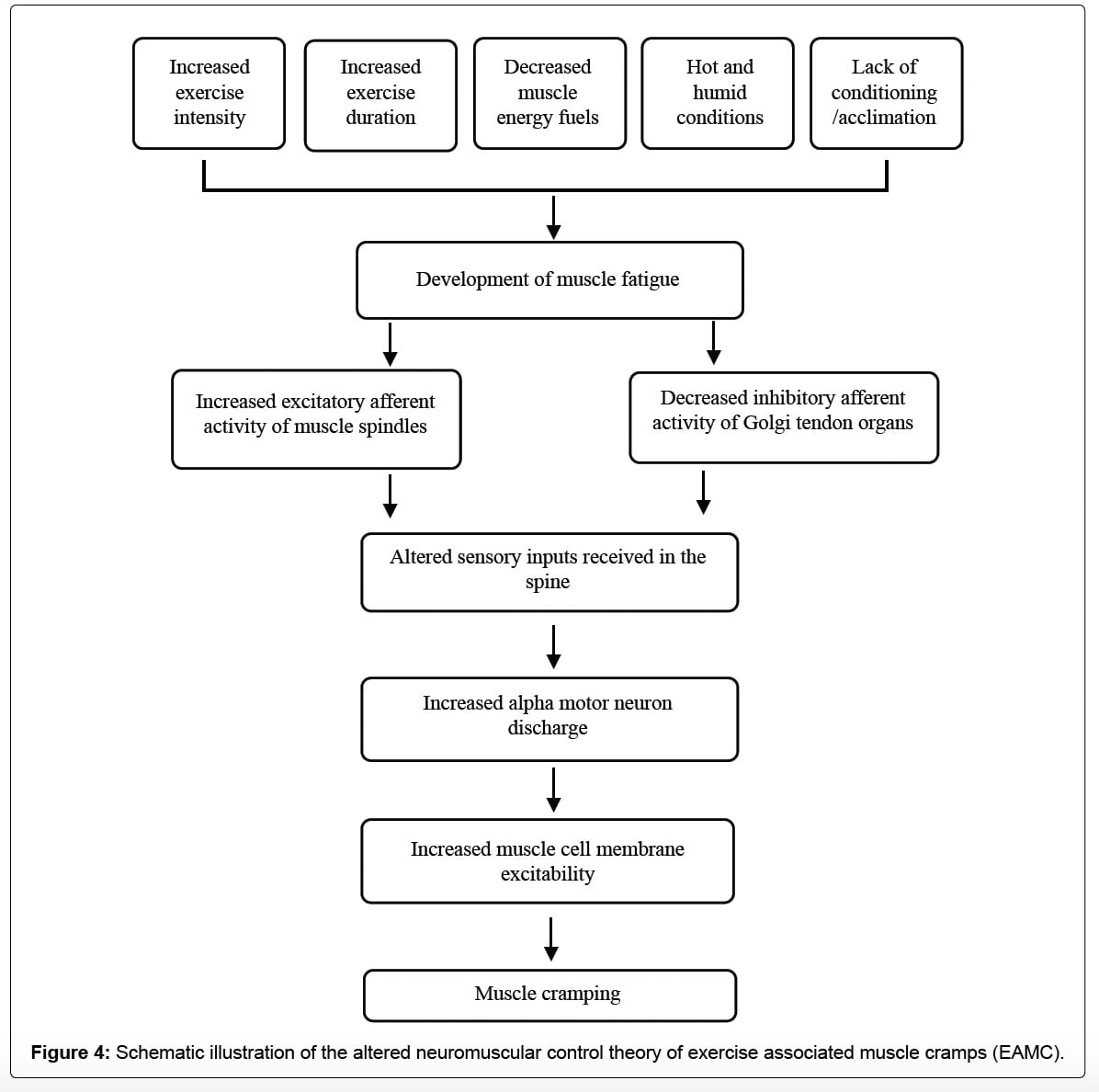
Multiple variables affect muscular fatigue and lead to an imbalance in communication between your muscle spindles and GTOs. This can cause incorrect information to be received by your nervous system (alpha motor neurons) and lead to extra messages to contract winning out and a muscle cramp occurring. Image from Qiu J, Kang J (2017).
Muscles that are most likely to experience EAMC are muscles that are contracting in a shortened position. This is particularly true of muscles that cross two joints, including your semitendinosus (one of the hamstrings), semimembranosus (another of the hamstrings), biceps femoris (the third of the hamstrings), rectus femoris (one of the quadriceps), and your gastrocnemius (one of the calf muscles). EAMC is not limited to biarticulated muscles of the legs, but they are the most common location reported by runners with EAMC. When muscles are contracting in an already shortened position, or in a small arc of movement, the GTO produces less inhibition than it does normally, because of altered muscle tension. This can be exacerbated if you have injuries or imbalances that decrease your range of motion (7). Giving your muscles an opportunity to lengthen, be it through your stride moving through a full range of motion, stretching outside of the occasionally compact ultra-shuffle, or in stopping and passively stretching the muscle, creating tension in the muscle increases the GTOs’ inhibitory input to the alpha motor neurons and relaxes the muscle (7, 12).
In 2010, Kevin Miller and his colleagues conducted the now-famous “Pickle Juice Study.” For decades, athletic trainers and coaches had anecdotally been prescribing pickle juice, apple-cider vinegar, and mustard to treat EAMC, but there was no concrete evidence as to why these substances seemed to quell cramps. Initially, there was some belief that it must be the sodium in the pickle juice that was helping to correct an electrolyte imbalance in the cramp sufferers. However, ingesting small volumes of pickle juice (30 to 60 milliliters) caused cramps to stop in as little time as 30 to 35 seconds. This response is much quicker than the amount of time it takes blood-electrolyte concentrations to change from drinking something. Miller induced cramps, using electrical stimulation, in hydrated participants and gave them either pickle juice or deionized water and saw the pickle juice dramatically cut down on cramp duration compared with deionized water or without any fluids ingested. They concluded that, while they were unsure which ingredient in pickle juice abated cramps so quickly (likely acetic acid/vinegar), it must trigger a neural reflex in the mouth, oropharynx, or esophagus quickly after ingestion, which in turn reduces alpha motor neuron activity to the cramping muscle (13). The conclusion from this one study opened up a new area of EAMC research, and has driven several new products to the market.
It has quickly caught on in the scientific community that the ingredients in pickle juice may very likely be stimulating what are known as transient receptor potential (TRP) channels. TRP channels are ion channels, excitable cells that convert chemical or mechanical messages into electrical signals, that help to mediate a variety of different sensations like pain, different tastes, hot, cold, and pressure. I first learned of TRP channels in my graduate thermophysiology course primarily as we focused on how the body perceives different temperatures. Many of these TRP channels can be activated by molecules found in spices such as capsaicin (part of chili peppers), menthol (part of mint), cinnamaldehye (part of cinnamon), shogaol (part of ginger), and allyl isothiocyanate (part of mustard). These relationships to particular channels are shown in the image below (14).
Researchers have become particularly interested in the TRPA1 and TRPV1 channels as they are found in the mucosal linings of the oropharyngeal region, or more specifically the mouth, oropharynx, esophagus, and stomach. With how fast-acting the acetic acid was on EAMC during the pickle-juice study, the presence of these channels above the stomach gives the theory some legs to stand on (15, 16).
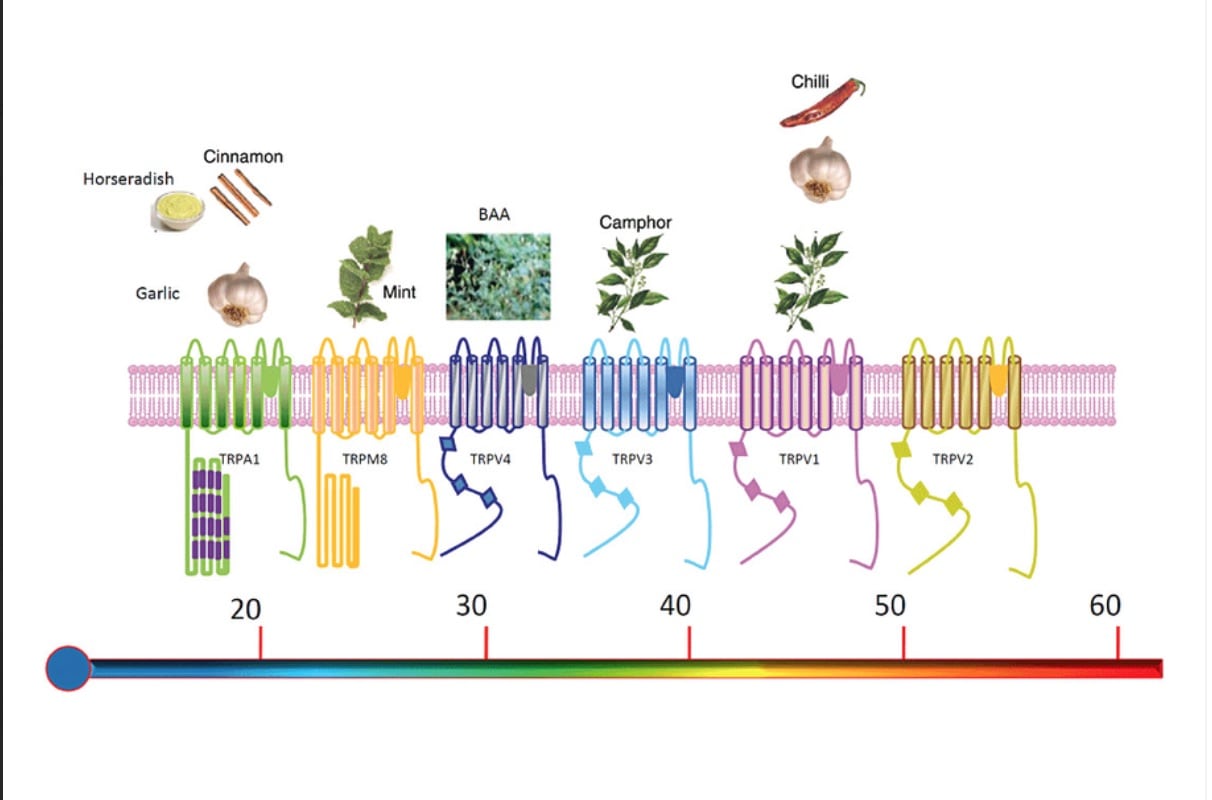
From left to right, the scale ranks TRP channels on the temperatures they are associated with from cold to hot. These same TRP channels also respond to chemicals in the food also shown on the chart. Image from Vay, L., Gu, C., & Mcnaughton, P. A. (2012).
What this means is that strong sensory stimuli activated at these specific TRP channels, by a TRP agonist, or activators for each channel, like capsaicin, can potentially cause the alpha motor neurons to become less excited which would in turn diminish or stave off a cramp (16). There are two possible scenarios being considered here: 1) pre-ingestion of a TRP agonist will increase the threshold one has to reach in order to cramp and keep the individual out of a cramp-prone state for longer, and 2) ingestion of a TRP agonist at onset of a cramp will ‘trip’ our electrical wiring, causing our muscle spindles and GTOs to work in harmony once again by decreasing the excitability of our alpha motor neurons.
This brings us to my favorite word of 2018: ‘bio-plausible.’ Bio-plausible arguably could mean something that adheres to biological reality as we presently understand things to work. Bio-plausible ideas, techniques, and specifically products are ones that are based on how we believe something should work at a mechanism level, in this case physiologically, but remains unproven. Or rather has yet to build concrete, thoroughly validated, evidence. Things that are bio-plausible, in essence, should work, at least… in theory, and sport is the perfect place for bio-plausible ideas as sport spurs innovation. A lot of great ideas start as merely bio-plausible but it takes years of trying to disprove such theories (because that’s how the scientific process works) that make them reliable, evidence-based, fact.
There a few products on the market targeting the exact theory that a strong orally ingested TRP agonist will help to prevent or abate cramps once they start. Some of these products include ACV Cramp Cure and Caleb Treeze Organic Farm Leg and Foot Cramps Liquid that are both formulated with apple-cider vinegar (acetic acid), garlic, and ginger. And more popularly, HotShot, which includes the TRP agonists capsaicin, ginger, and cinnamon.
As of 2017, there have been a few studies done on TRP agonists and EAMC. However, there is more work to be done as two of the three major studies published on TRP agonists and EAMC have been done by the creators of HotShot, a company called Flex Pharma (16, 18). Just as I’ve asked my students to do when evaluating research articles, I ask you to remain objective, unbiased, and open-minded when it comes to evaluating peer-reviewed journal articles. You’ll find that at the bottom of articles, after a limitations section, conflicts of interest, if any, are generally listed.
In both studies (16, 18), subjects were given an orally administered blend of TRP agonists and then had cramps both electrically induced or voluntary cramping via maximum isometric contractions of five to 90 seconds. The studies both evaluated time to onset of cramp, time to cramp subsiding, and intensity of cramping in small foot muscles. They found that ingesting the TRP agonists increased the time contracting and force output pre-cramp initiation, and a shortened time during cramping (16). In the former study, not only did they find that the intensity of the cramp was reduced by threefold, but that orally ingesting a TRP agonist could increase an individuals’ cramp-threshold frequency for up to six to eight hours after ingestion. The cramp-threshold frequency is the lowest electrical-stimulation frequency that can produce a cramp, which is inversely related to your susceptibility to cramping (19).
In a later, more recent study conducted also using HotShot’s proprietary blend of TRP agonists, researchers once again brought on electrically induced cramps after ingesting the TRP-agonist-containing supplement, but rather than focusing on the foot they chose a larger lower-leg muscle, the medial gastrocnemius (one of the calf muscles). Similar to the previous studies, they were able to elicit a quicker attenuation of the cramp and its intensity but they could not repeat the same prolonged effects. They found that the largest increase in cramp-threshold frequency was only present for approximately 15 minutes post-ingestion but was gone by their retest times of four hours, eight hours, and 24 hours post-ingestion (19).
So what does that all mean? It means that there is very likely an ability to activate TRP channels and create a stimulation that will suppress the alpha motor neurons hyperexcitability and alleviate an existing muscle cramp. It would also appear that at this time we do not know for certain if these same TRP agonists can produce a preemptive effect on alpha motor neurons and keep us out of a cramp-prone state in advance. I would add that at this time more research needs to be conducted on this and other products containing TRP agonists like mustard, apple-cider vinegar, menthol, and more. We are just at the beginning stages of understanding the complexities of TRP channels, the electrical component of EAMC, and their physiological intricacies.
To finish, I will add that the body is complicated and that we, and surely I, do not know everything. And as stated by Schwellnus & Hoffman this past fall, it is still apparent that EAMC is made up of a complex set of underlying variables that makes the idea that there is any one single treatment or prevention strategy for EAMC implausible (1). Which leads me to our takeaways:
- Anecdote is not science. The brain is incredibly powerful, and placebos can have very real effects on physiological symptoms and performance. It doesn’t mean that something will not work, but the reliability of such methodologies remain unproven.
- We should not completely write off proper hydration and electrolyte intake even if reliable evidence to support this practice as a foolproof preventative strategy for EAMC does not exist. Improperly done dehydration and electrolyte imbalance may be compounding variables to fatiguing muscles (6).
- Train yourself specifically for the event you are undertaking. It’s thought that when the demand you put on your muscles does not match up with the training you’ve done, you are more susceptible to cramping (5). This goes for being properly heat acclimated, altitude acclimated, going faster than you train, and being prepared for the types of terrain you will be competing on. Nothing can protect you from being underprepared for an event, not even the powerful miscalculation of our own limitations.
- Work on form, mobility, and range of motion. Muscles most affected by EMAC are those that are confined to a small arc of motion, in a shortened state, and used repetitively. Avoid heavy braking and focus on manipulating your stride length (in training for race day) so that you can maintain adequate hip and knee flexion and extension.
- Make mobility part of your fitness and race strategy. Address your own body’s imbalances through stretching, physical therapy, yoga, and massage. There is evidence that preemptive self-care can make a difference come race day (7).
- Fuel adequately. Glycogen depletion and inadequate fuel for your working muscles can lead to premature muscle fatigue and increase your risk of cramping.
- Learn to recognize your body’s pre-cramping state and respond accordingly. Slowing down or stopping to stretch cramp-prone muscles could save you from that DNF or crawling into the next aid station.
- Just like you practice your hydration and nutrition plan before your A race, consider practicing your cramping protocol. Experiment with different TRP agonist products or concoctions. Research has yet to be done on possible negative side effects of many of the ingredients used as TRP agonists in these products, such as thermoregulation changes and gastrointestinal distress.
- Be reflective. Evaluate the training or race-day scenarios that may have brought you to your knees. What factors may have combined to lead to an over-fatigued state? For me personally it’s been a journey of rejiggering my biomechanics and imbalances.
[Author’s Note: The author of this article reports no conflicts of interest with this article. She is unsponsored and not financially supported by any of the companies involved in the research presented above and approached the writing of this article from an unbiased research-minded place.]
Call for Comments (from Meghan)
- Exercise-associated cramps, do you get them or not?
- If you are, as Corrine says, a cramper, have you sorted out what underlying issues have exacerbated them in you? And, have you found anything that helps you end them?
References
- Schwellnus, M. P., & Hoffman, M. D. (2017). Transient receptor potential channels and exercise-associated muscle cramping: A tale of multiple complexities. Muscle & Nerve, 56(3), 355-357. doi:10.1002/mus.25668
- Schwellnus MP, Nicol J, Laubscher R, Noakes TD. Serum electrolyte concentrations and hydration status are not associated with exercise associated muscle cramping (EAMC) in distance runners. Br J Sports Med. 2004; 38:488–492. PubMed doi:10.1136/bjsm.2003.007021
- Schwellnus MP, Drew N, Collins M. Increased running speed and previous cramps rather than dehydration or serum sodium changes predict exercise-associated muscle cramping: a prospective cohort study in 210 Ironman triathletes. Br J Sports Med. 2011; 45:650–656. PubMed doi:10.1136/bjsm.2010.078535
- Sulzer NU, Schwellnus MP, Noakes TD. Serum electrolytes in Ironman triathletes with exercise-associated muscle cramping. Med Sci Sports Exerc. 2005; 37:1081–1085. PubMed doi:10.1249/01.mss.0000169723.79558.cf
- Schwellnus MP, Allie S, Derman W, Collins M. Increased running speed and pre-race muscle damage as risk factors for exercise-associated muscle cramps in a 56 km ultra-marathon: a prospective cohort study. Br. J. Sports Med. 2011; 45: 1132–6.
- Buskard, A.N. (2014). Cramping in Sports. Strength and Condition Journal, 36(5), 44-52. doi:10.1519/ssc.0000000000000091
- Qiu J, Kang J (2017) Exercise Associated Muscle Cramps – A Current Perspective. Scientific Pages Sports Med 1(1):3-14.
- Stofan, J., Zachwieja, J., Horswill, C., Murray, R., Anderson, S., & Eichner, E. (2005). Sweat and sodium losses in NCAAfootball players: A precursor to heat cramps? International Journal of Sport Nutrition and Exercise Metabolism, 15, 641–652.
- Braulick, K., Miller, K., Albrecht, J., Tucker, J., & Deal, J. (2013). Significant and serious dehydration does not affect skeletal muscle cramp threshold frequency. British Journal of Sports Medicine, 47, 710–714. doi:1136/bjsports-2012-091501
- Hoffman, M. D., & Stuempfl, K. J. (2015). Muscle Cramping During a 161-km Ultramarathon: Comparison of Characteristics of Those with and Without Cramping Sports Medicine – Open 1(1). Doi:10.1186/s40798-015-0019-7
- Schwellnus, M. P., Drew, N., & Collins, M. (2008). Muscle Cramping in Athletes—Risk Factors, Clinical Assessment, and Management. Clinics in Sports Medicine,27(1), 183-194. doi:10.1016/j.csm.2007.09.006
- Schwellnus M. Cause of exercise associated muscle cramps (EAMC)– altered neuromuscular control, dehydration or electrolyte depletion? Br J Sports Med 43: 401–408, 2009
- Miller, K. C., Mack, G. W., Knight, K. L., Hopkins, J. T., Draper, D. O., Fields, P. J., & Hunter, I. (2010). Reflex Inhibition of Electrically Induced Muscle Cramps in Hypohydrated Humans. Medicine & Science in Sports & Exercise,42(5), 953-961. doi:10.1249/mss.0b013e3181c0647e
- Vay, L., Gu, C., & Mcnaughton, P. A. (2012). The thermo-TRP ion channel family: properties and therapeutic implications. British Journal of Pharmacology,165(4), 787-801. doi:10.1111/j.1476-5381.2011.01601.x
- Alvarez-Berdugo D, Rofes L, Farre R, Casamitjana JF, Enrique A, Chamizo J, Padron A, Navarro X, Clave P. Localization and expression of TRPV1 and TRPA1 in the human oropharynx and larynx. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 2016;28(1):91-100.
- Craighead DH, Shank SW, Gottschall JS, Passe DH, Murray B, Alexander LM, et al. Ingestion of transient receptor potential channel agonists attenuates exercise-induced muscle cramps. Muscle Nerve 2017;56:379–385.
- Murray, D., Miller, K. C., & Edwards, J. E. (2016). Does a Reduction in Serum Sodium Concentration or Serum Potassium Concentration Increase the Prevalence of Exercise-Associated Muscle Cramps? Journal of Sport Rehabilitation,25(3), 301-304. doi:10.1123/jsr.2014-0293
- Short G, Hegarty B, MacKinnon R, Bean B, Westphal C, Cermak J (2015) Orally-administered TRPV1 and TRPA1 activators inhibit electrically-induced muscle cramps in normal healthy volunteers (S17.003). Neurology 84(14 Suppl).
- Behringer, M., Nowak, S., Leyendecker, J., & Mester, J. (2017). Effects of TRPV1 and TRPA1 activators on the cramp threshold frequency: a randomized, double-blind placebo-controlled trial. European Journal of Applied Physiology,117(8), 1641-1647. doi:10.1007/s00421-017-3653-6
- Parisi L, Pierelli F, Amabile G, et al. Muscular cramps: proposals for a new classification. Acta Neurol Scand 2003;107:176–86.
